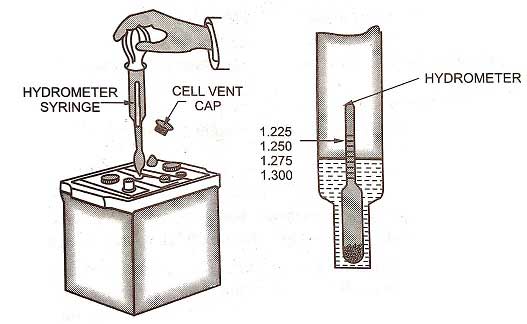
SPECIFIC GRAVITY TEST
While the chemical reactions are taking place in the battery during discharge, the electrolyte becomes dilute due to the formation of water. The solution of water and acid consists of 65% water and 35% sulfuric acid. The specific gravity of the electrolyte depends on this 65% to 35% ratio for the required chemical reaction to take place.The proportion of water goes on increasing as the discharging continues. The relative amounts of water and acid are determined by the specific gravity test. This is done by the use of a hydrometer contained in a syringe. The electrolyte is drawn up into the syringe by the bulb and the hydrometer float sinks to a greater or lesser extent according to the amount of sulphuric acid in the electrolyte.
 |
| Hydrometer Float |
Operation
If the hydrometer leads 1.280, it indicates that the liquid is 1.280 times as heavy as water and at the reading, the battery is fully charged. The readings of the hydrometer show the condition of the battery at 80°F (27°C) in accordance with the following table: |
| Specific Gravity Test by Hydrometer |
READING
|
CONDITION
|
1.220 - 1.230
1.200 - 1.210
1.175 - 1.185
1.150 - 1.160
1.125 - 1.135
1.100 - 1.110
|
Fully Charged
¾ charged
½ charged
¼ charged
Very little charged
Discharged
|
The scale of the hydrometer is read on the stem at the surface of the electrolyte when the hydrometer is floating in it. The specific gravity of the electrolyte is affected by its temperature. Thus, the temperature, of the electrolyte is also recorded by a thermometer and then the specific gravity reading is accordingly.
The specific gravity test should not be made while the battery is gassing. The test should also not be made immediately after the water has been added to adjust the electrolyte level, otherwise, this would affect the reading.
 |
| Specific Gravity Test |

ConversionConversion EmoticonEmoticon